Best Lewis Structure For Ch2cl2
Dichloromethane (CHiiCl2) Lewis Structure
Dichloromethane (CH2Cl2) contains one carbon cantlet, two hydrogen atoms and 2 chlorine atoms. In the lewis structure of CH2Cl2, carbon atom is located as the center atom and other atoms have fabricated bonds with carbon atom. Both chlorine cantlet has three lone pairs and carbon cantlet does not has lone pairs.
CH2Cltwo lewis structure
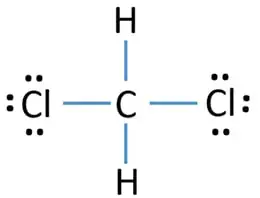
Both hydrogen atoms and both chlorine atoms have made single bonds with carbon atom. Therefore, at that place are four singe bonds effectually carbon atom. Both chlorine atom has 3 lone pairs in their valence shells.
Dichloromethane (CHiiCl2)
A colourless liquid at room temperature and a highly volatile substance. Besides as, it is a toxic chlorohydrocarbons chemical compound. Now, we tin report, how dichloromethane'southward lewis structure is fatigued step past step in this tutorial.
Steps of drawing lewis construction of CH2Cl2
When we describe a lewis structure, at that place are several guidelines to follow. Number of steps can be changed co-ordinate the complexity of the molecule or ion. Even so those all steps are mentioned and explained in item in this tutorial for your knowledge.
- Find total number of electrons of the valance shells of carbon, hydrogen and chlorine atoms
- Decide total electrons pairs existing equally lone pairs and bonds
- Deciding center atom selection
- Marking lone pairs on atoms
- Marker charges on atoms if at that place are charges.
- Check the stability and minimize charges on atoms past converting lone pairs to bonds to obtain best lewis structure.
Total number of electrons of the valance shells of CHiiCl2
There are 3 elements in dichloromethane; carbon, hydrogen and chlorine. Hydrogen is a group IA element in the periodic table and only has one electron in its last shell (valence trounce).Carbon is a grouping IVA element in the periodic table and has four electrons in its last shell. Too, Chlorine is a group VIIA element in the periodic tabular array and contains seven electrons in its last beat out. Now, we know how many electrons are there in valence shells of each hydrogen, carbon and chlorine atoms and tin can calculate total number of electrons in their valence shells.
- valence electrons given by hydrogen atom = 1 * 2 = 2
- valence electrons given by carbon atom = iv * 1 = 4
- valence electrons given past chlorine atoms = 7 * ii = xiv
- Full valence electrons = 2 + four + 14 = 20
Total valence electrons pairs
Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells
Total electron pairs are adamant by dividing the number total valence electrons by two. For, CH2Cl2, total pairs of electrons are 10 in their valence shells.
Heart atom of CHtwoCl2
Hydrogen cantlet cannot be a centre atom because hydrogen atom can just go on two electrons in last shell.
Because, chlorine tin bear witness higher valence (7) than carbon (iv), nosotros tin think chlorine should be the center atom. But, due to carbon is more electropositive than chlorine and because stability of molecule, carbon is the center atom. Basic skeletal of CH2Cl2 is shown below.

Mark lone pairs on atoms
Afterwards determining the center cantlet and skeletal of CH2Cl2 molecule, we can start to mark solitary pairs on atoms. Remember that, there are total of ten electron pairs.
- In that location are already four bonds in the drawn skeletal. So, at present only six electron pairs are remaining to mark as lone pairs.
- Usually, those remaining electron pairs should be started to mark on outside atoms. Therefore, we can start to mark those remaining electrons pairs on chlorine atoms because each hydrogen atom aleady has two electrons in their valence shell. Therefore, chlorine atom will take 3 lone pair. Then, all remaining six electron pairs are marked.
- Now, there are no more alone pairs to mark on carbon atom.

Marking charges on atoms
There are no charges on atoms in above structure.
Check the stability and minimize charges on atoms past converting lone pairs to bonds
Because 3 are no charges on atoms in higher up CH2Cl2 structure, we do not need to do the step of reducing charges on atoms by converting lone pairs to bonds. That means, we have obtained the best structure for dichloromethane lewis construction.
Questions
Does dichloromethane has charges?
At that place is no overall charge in dichloromethane molecule. Also, individual atoms practice not have charges. And so, dichloromethane is a neutral molecule.
Best Lewis Structure For Ch2cl2,
Source: https://www.chemistryscl.com/general/CH2Cl2-dichloromethane-lewis-structure/
Posted by: taylorencell1939.blogspot.com


0 Response to "Best Lewis Structure For Ch2cl2"
Post a Comment